The UDI (Unique Device Identification) system, which is developed to increase the traceability and patient safety of medical devices, has become compulsory with various regulations worldwide. This system allows the unique identification of devices and provides transparency in all processes from production to use. The UDI code consists of two basic components: the device identifier (UDI-DI) and the production identifier (UDI-PI).
Contents
Basic components of the UDI code
Source: tritonstore.com.au
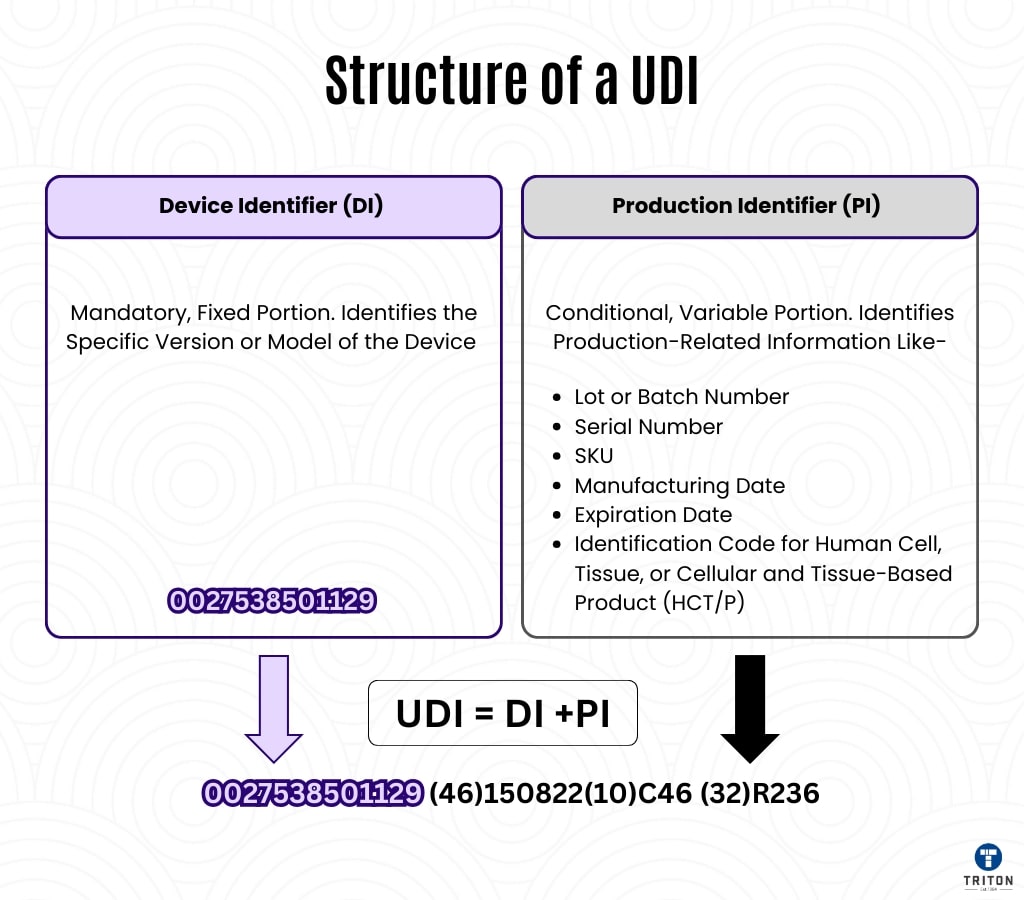
Device identifier (UDI-DI)
- It is the fixed part of the UDI code and is specific to the model or type of the device.
- Defines the manufacturer, the trademark, model and type of the device.
- It does not change after the release of the device, so it is critical for traceability.
- It is offered in the form of barcode or QR code in the labels to quickly define the device. (For example, the UDI-DI code in a blood sugar measuring device remains the same in all versions of the device.)
Production Description (UDI-PI)
- Contains the production details and variable data of the device.
- Information such as serial number, lot number, production date and expiration date are included in the UDI-PI code.
- It provides a unique definition for each unit, especially on disposable or short -lived devices.
This information provides a quick and effective process when the products need to be recovered.
You may be interested in: Date Coding on Egg for Egg Manufacturers
Application of UDI code in medical devices
Labeling and direct marking
The labeling contains the UDI code placed on the device or in the packaging. The code is presented in both readable formats by the machine (barcode, QR code) and by human beings. Direct marking is especially preferred on reusable devices. (For example, a permanent UDI code can be marked by laser scraping method on a surgical instrument.)
Special marking for reusable devices
Reusable devices, disinfection and sterilization requires the code to be found permanently on the device during operations such as sterilization. This allows easy monitoring of devices in hospital environments. The UDI marking must be done in a way that does not affect the usability of the device. [1]
Contribution of UDI Code to the Health System
UDI code is a revolutionary in terms of the traceability of medical devices. With this system, all stages of a device from production to use can be recorded. When wrong production or safety problems are detected, recall processes can be carried out rapidly. For example, if the devices produced in a certain lot need to be withdrawn, which products are on the market can be easily detected. [2]
The UDI code also contributes a great contribution to patient safety. It facilitates the verification of the authenticity of the devices and is an effective tool in the fight against fraud. In addition, since it provides integration with electronic health records, it provides detailed information about which patient uses which device. For example, it is possible to see the model and serial number of a patient's implant device directly in health recording.
International Standards and Regional Regulations
- Standards such as ISO 13485 and ISO 14971 regulate quality management and risk assessment of medical devices.
- In the USA, the FDA UDI system requires that the devices be saved in the database.
- In the European Union, the UDI system is applied within the scope of MDR (Medical Device Regulation) and IVDR (In vitro diagnostic regulation). [3]
You may be interested in: Electrojet solutions attracted attention at the Eurasian packaging fair
Last word
The UDI system has created a major transformation in the health sector by providing traceability of medical devices from production to patient use. This system not only increases patient safety, but is also used as an effective tool in the fight against fraud. Accelerating recalcision processes and providing the reliability of medical devices provides transparency and efficiency in health systems.
Electrojet , we are proud to offer inkjet coding machines and barcode printers fully compatible with the UDI system. Our products are designed to meet the needs of all production sectors, not only medical devices. UDI codes, serial numbers, production dates and the technologies we have developed to print the barcodes in a precision manner support businesses to adapt to regulatory requirements.

If you want to make UDI coding or barcode printing processes more efficient in your business, you can meet Electrojet's expert solutions. With our technological infrastructure and sectoral experience, we offer special solutions for all kinds of coding needs.
Contact us to learn the most suitable devices for your business and free .
References:
- [1]
- [2] https://tritonstore.com.au/what-is-a-udi/
- [3]
- [4]